Apr 26, 2017 27.0 g Explanation: The density of a substance tells you the mass of exactly one unit of volume of said substance. In your case, aluminium is said to have a density of density Al = 2.70 g.cm−3 This tells you that every 1 mL of aluminium has a mass of 2.70 g.
⏩SOLVED:The density of aluminum is 2.70 g / cm^3. A square piece of… | Numerade
The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil, 22.86 cm on a side is found to weigh 2.568 g. What is the thickness of the foil, in millimeters? 00:27. Aluminum has a density of 2.70 g/cm³. A piece of foil measures 10.4 cm by 5.75 cm and weighs 0.268 g. What is the thickness of the foil?
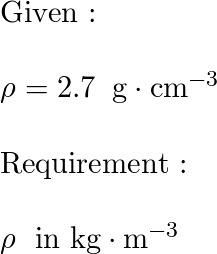
Source Image: quizlet.com
Download Image
The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil 22.86 cm on one side weighs 2.568 g. What is the thickness of the foil in mm? express your answer in decimal notation rounded to the correct number of significant figures. Problem 1.107QP: Obtain the difference in volume between two spheres, one of radius 5.61 cm, the other
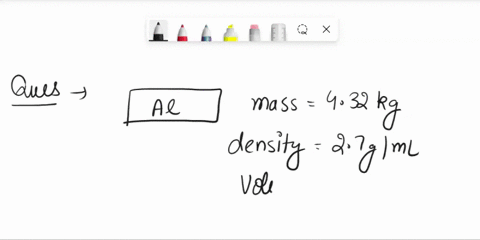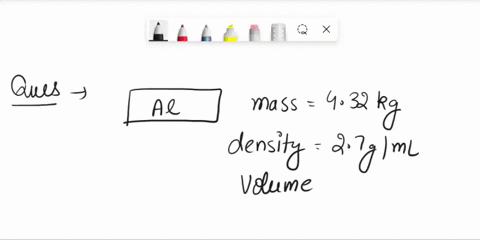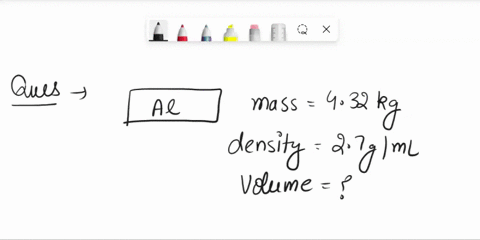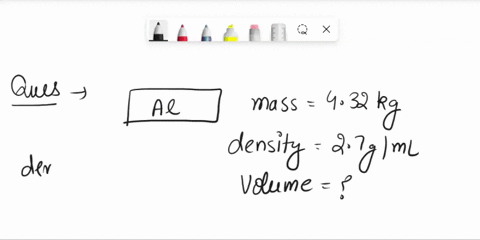
Source Image: numerade.com
Download Image
SOLVED: The density of aluminum is 2.70 g / cm^3 and its atomic mass ts 26.97 u. The electronic structure of aluminum is given in Table 7.4 (the energy difference between 3 The density of common metals such as iron is 7.87 g/cm3, mild steel is 7.85 g/cm3, 304 stainless steel is 8.0 g/cm3, aluminum is 2.7g/cm3, copper is 8.93 g/cm3, gold is 19.3 g/cm3, silver is 10.49 g/cm3, for more metals, please view the metal density chart and table below. Common & Less Common Metal Density Chart / Table

Source Image: brainly.com
Download Image
The Density Of Aluminum Is 2.70 G Cm3
The density of common metals such as iron is 7.87 g/cm3, mild steel is 7.85 g/cm3, 304 stainless steel is 8.0 g/cm3, aluminum is 2.7g/cm3, copper is 8.93 g/cm3, gold is 19.3 g/cm3, silver is 10.49 g/cm3, for more metals, please view the metal density chart and table below. Common & Less Common Metal Density Chart / Table Question: The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil 22.86 cm on one side weighs 2.568 g. What is the thickness of the foil in mm? Express your answer in scientific notation with 3 significant figures. Do not include the units The accepted format for scientific notation answers in Canvas is m∗10∧n, where m
Metal Density (g/cm3) Aluminum 2.70 Zinc 7.13 Iron 7.87 Copper 8.96 6. Use the table above to – brainly.com
1 Answer Chuck W. Jun 7, 2016 The mass of an object is equal to the product of its density and volume. Check to ensure that the units cancel properly. Explanation: 2.70 g cm3 ×1.50cm3 = 4.05g Answer link The mass of an object is equal to the product of its density and volume. SOLVED: The density of aluminum is 2.70 g / cm^3. What volume does 2.00 kg occupy?

Source Image: numerade.com
Download Image
Solved Aluminium has a density of 2.70 g/cm How many moles | Chegg.com 1 Answer Chuck W. Jun 7, 2016 The mass of an object is equal to the product of its density and volume. Check to ensure that the units cancel properly. Explanation: 2.70 g cm3 ×1.50cm3 = 4.05g Answer link The mass of an object is equal to the product of its density and volume.
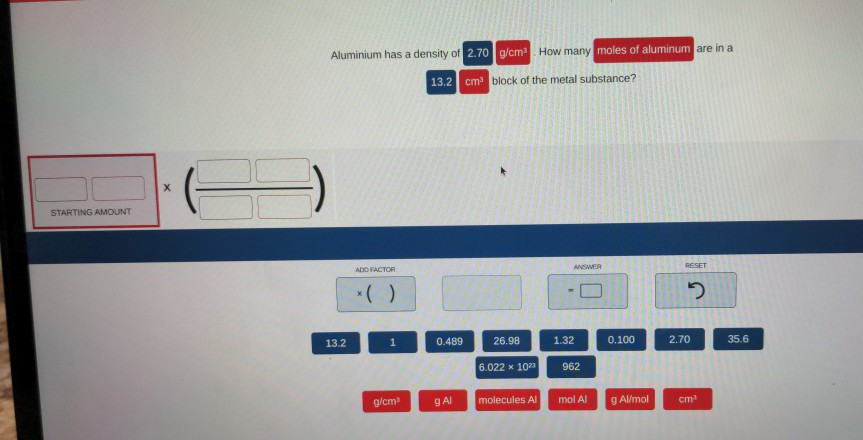
Source Image: chegg.com
Download Image
⏩SOLVED:The density of aluminum is 2.70 g / cm^3. A square piece of… | Numerade Apr 26, 2017 27.0 g Explanation: The density of a substance tells you the mass of exactly one unit of volume of said substance. In your case, aluminium is said to have a density of density Al = 2.70 g.cm−3 This tells you that every 1 mL of aluminium has a mass of 2.70 g.

Source Image: numerade.com
Download Image
SOLVED: The density of aluminum is 2.70 g / cm^3 and its atomic mass ts 26.97 u. The electronic structure of aluminum is given in Table 7.4 (the energy difference between 3 The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil 22.86 cm on one side weighs 2.568 g. What is the thickness of the foil in mm? express your answer in decimal notation rounded to the correct number of significant figures. Problem 1.107QP: Obtain the difference in volume between two spheres, one of radius 5.61 cm, the other
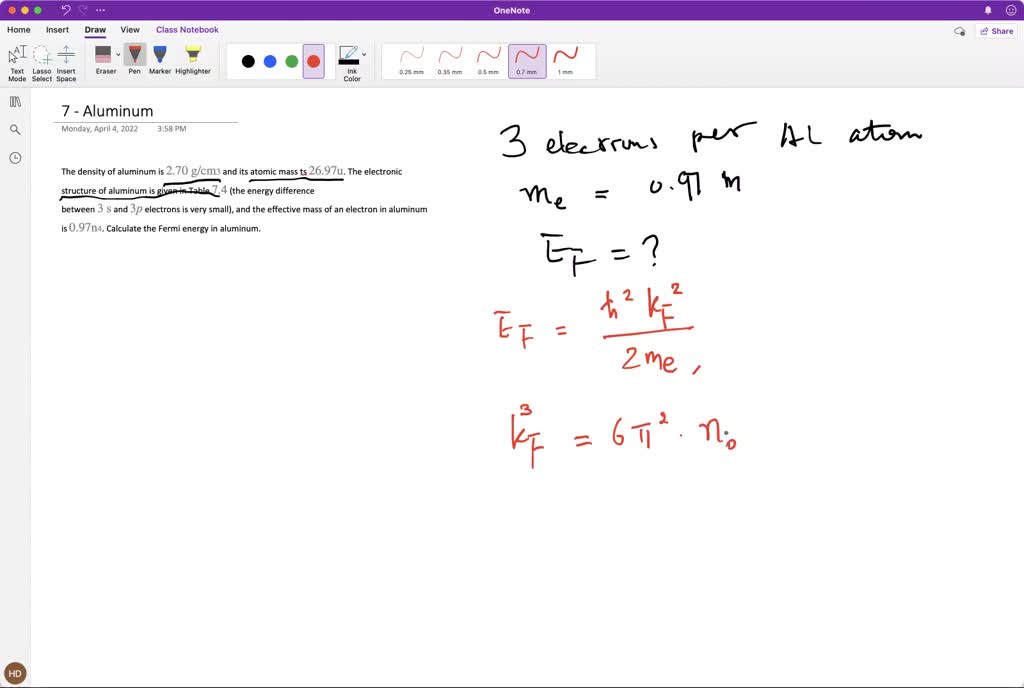
Source Image: numerade.com
Download Image
How to Determine Metal Density – Canadian Conservation Institute (CCI) Notes 9/10 – Canada.ca A piece of aluminum has density 2.70 g/cm3 and mass 775 g. The aluminum is submerged in a container of oil of density 0.650 g/cm3. A spring balance is attached with string to the piece of aluminum. What reading will the balance register in grams (g) for the submerged metal? Click the card to flip 👆 588 g Click the card to flip 👆 1 / 75 Flashcards

Source Image: canada.ca
Download Image
Density Buoyant Force and Pressure | PDF | Buoyancy | Density The density of common metals such as iron is 7.87 g/cm3, mild steel is 7.85 g/cm3, 304 stainless steel is 8.0 g/cm3, aluminum is 2.7g/cm3, copper is 8.93 g/cm3, gold is 19.3 g/cm3, silver is 10.49 g/cm3, for more metals, please view the metal density chart and table below. Common & Less Common Metal Density Chart / Table

Source Image: scribd.com
Download Image
If the density of aluminium is 2700 kg m^(-3), then its value in the CGS system is | CLASS 9 | … – YouTube Question: The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil 22.86 cm on one side weighs 2.568 g. What is the thickness of the foil in mm? Express your answer in scientific notation with 3 significant figures. Do not include the units The accepted format for scientific notation answers in Canvas is m∗10∧n, where m
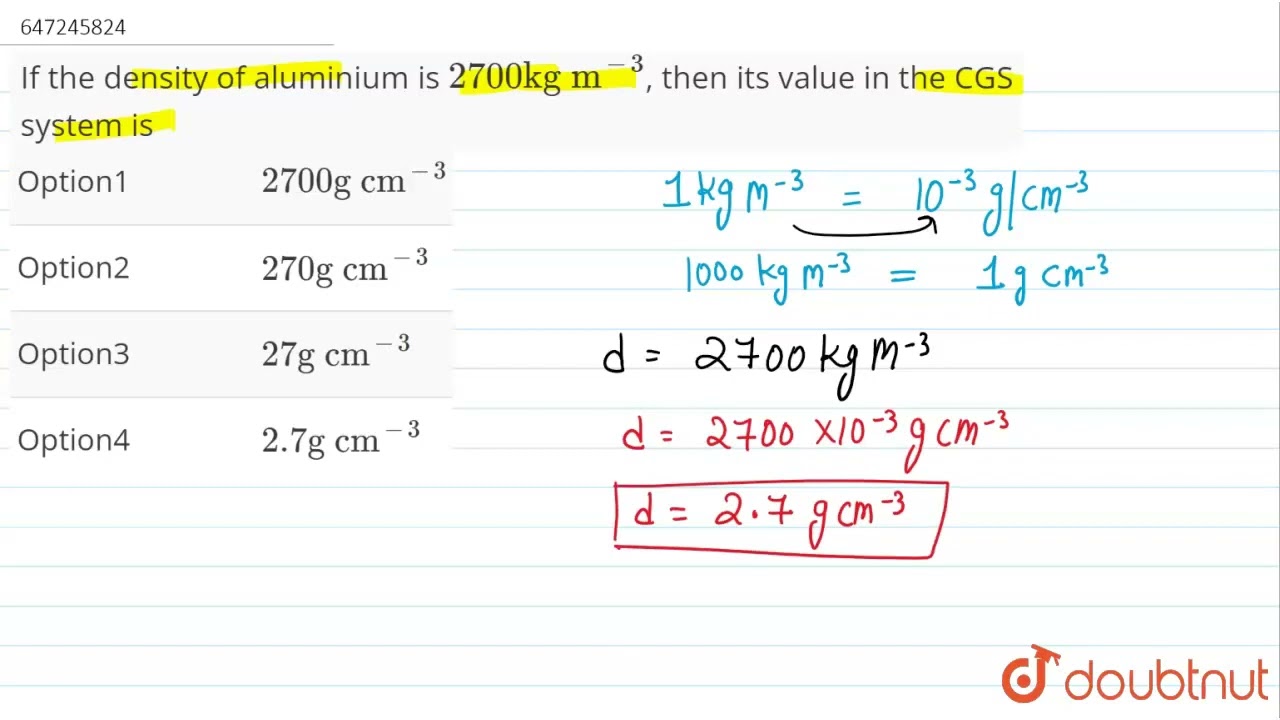
Source Image: youtube.com
Download Image
Solved Aluminium has a density of 2.70 g/cm How many moles | Chegg.com
If the density of aluminium is 2700 kg m^(-3), then its value in the CGS system is | CLASS 9 | … – YouTube The density of aluminum is 2.70 g/cm3. A square piece of aluminum foil, 22.86 cm on a side is found to weigh 2.568 g. What is the thickness of the foil, in millimeters? 00:27. Aluminum has a density of 2.70 g/cm³. A piece of foil measures 10.4 cm by 5.75 cm and weighs 0.268 g. What is the thickness of the foil?
SOLVED: The density of aluminum is 2.70 g / cm^3 and its atomic mass ts 26.97 u. The electronic structure of aluminum is given in Table 7.4 (the energy difference between 3 Density Buoyant Force and Pressure | PDF | Buoyancy | Density A piece of aluminum has density 2.70 g/cm3 and mass 775 g. The aluminum is submerged in a container of oil of density 0.650 g/cm3. A spring balance is attached with string to the piece of aluminum. What reading will the balance register in grams (g) for the submerged metal? Click the card to flip 👆 588 g Click the card to flip 👆 1 / 75 Flashcards