or moles Al to grams Percent composition by element Element: Aluminium Symbol: Al Atomic Mass: 26.981538 # of Atoms: 1 Mass Percent: 100.000% Calculate the molecular weight of a chemical compound Enter a chemical formula: Browse the list of common chemical compounds. Recommended videos Powered by AnyClip AnyClip Product Demo 2022
2022 Paula’s Choice Review
26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) Solving for the atomic mass of Aluminium (Al) Need to know the atomic mass of a Aluminium molecule?

Source Image: autosport.com
Download Image
Al (Aluminium/Aluminum) 26.9815386. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Al: Molar Mass (g/mol) Al (Aluminium/Aluminum) 1 × 26.9815386 = 26.9815386. 4.

Source Image: tubomart.com
Download Image
How to Make Easy Homemade Pasta Dough – The Burnt Butter Table Definitions Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.

Source Image: youtube.com
Download Image
What Is The Molar Mass Of Al
Definitions Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol. 6: Chemical Composition
What is the molar mass of aluminum? – YouTube
Symbol: Al Atomic Mass: 26.981538 # of Atoms: 2 Mass Percent: 35.938%. Element: Sulfur Symbol: S Atomic Mass: 32.065 # of Atoms: 3 Mass Percent: 64.062%. Calculate the molecular weight of a chemical compound. … If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. … Deep Multi-task Learning and Real-time Personalization for Closeup Recommendations | by Pinterest Engineering | Pinterest Engineering Blog | Medium
Source Image: medium.com
Download Image
Dove Beauty 0% Aluminum Pomegranate & Lemon Verbena Women’s Deodorant Stick – 2.6oz : Target Symbol: Al Atomic Mass: 26.981538 # of Atoms: 2 Mass Percent: 35.938%. Element: Sulfur Symbol: S Atomic Mass: 32.065 # of Atoms: 3 Mass Percent: 64.062%. Calculate the molecular weight of a chemical compound. … If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. …

Source Image: target.com
Download Image
2022 Paula’s Choice Review or moles Al to grams Percent composition by element Element: Aluminium Symbol: Al Atomic Mass: 26.981538 # of Atoms: 1 Mass Percent: 100.000% Calculate the molecular weight of a chemical compound Enter a chemical formula: Browse the list of common chemical compounds. Recommended videos Powered by AnyClip AnyClip Product Demo 2022

Source Image: healthline.com
Download Image
How to Make Easy Homemade Pasta Dough – The Burnt Butter Table Al (Aluminium/Aluminum) 26.9815386. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Al: Molar Mass (g/mol) Al (Aluminium/Aluminum) 1 × 26.9815386 = 26.9815386. 4.

Source Image: theburntbuttertable.com
Download Image
Scientists Say: Periodic table Jan 18, 2024The SI unit of molar mass is kg/mol, but the g/mol unit is more commonly used. Molar mass vs. molecular weight It may seem as though the two quantities mean the same thing, but this is not true. Molecular weight and molar mass are different quantities, although numerically equal:
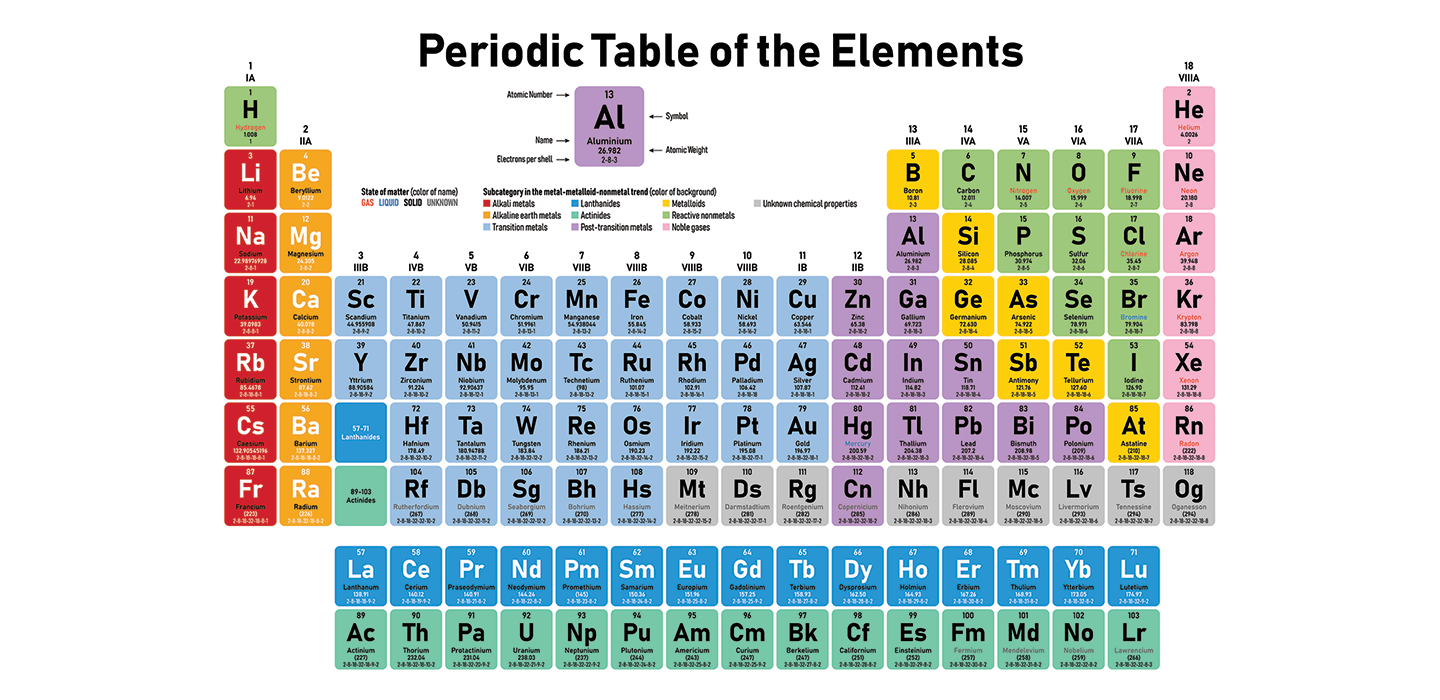
Source Image: snexplores.org
Download Image
Carbon 60 and Himalayan Shilajit: A Synopsis | by Glo Power Tea | Medium Definitions Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.

Source Image: glopowertea.medium.com
Download Image
An Effortless Daily, Weekly, and Monthly Guide to Better Skin 6: Chemical Composition

Source Image: healthline.com
Download Image
Dove Beauty 0% Aluminum Pomegranate & Lemon Verbena Women’s Deodorant Stick – 2.6oz : Target
An Effortless Daily, Weekly, and Monthly Guide to Better Skin 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) Solving for the atomic mass of Aluminium (Al) Need to know the atomic mass of a Aluminium molecule?
How to Make Easy Homemade Pasta Dough – The Burnt Butter Table Carbon 60 and Himalayan Shilajit: A Synopsis | by Glo Power Tea | Medium Jan 18, 2024The SI unit of molar mass is kg/mol, but the g/mol unit is more commonly used. Molar mass vs. molecular weight It may seem as though the two quantities mean the same thing, but this is not true. Molecular weight and molar mass are different quantities, although numerically equal: